Objective
The purpose of the experiment was to separate the individual elements of specific product, Phensuprin using the acid/base extraction process. This analgesic product was contained aspirin (acetylsalicylic acid), acetanilide, and sucrose. These compounds have different solubility. The extraction was used because one solute was more soluble in the solvent than another was.
Physical Properties
-

Molecular structure Sucrose:1
Form: White crystalline powder
Molecular formula: C12H22O11
Molecular weight: 342.3
Melting point: 185-1870 C
Boiling point: decomposes
Water solubility at 200 C: 1970 g/L
Hazard: Irritant -

Molecular structure Acetylsalicylic Acid:2
Form: White crystals
Molecular formula: C9H8O4
Molecular weight: 180.16
Melting point: 134-1360 C
Boiling point: 1400 C
Water solubility at 200 C: 3.3 g/L
Hazards: poisonous, irritant, and combustible -

Molecular structure Dichloromethane: 3
Form: Colorless liquid
Molecular formula: CH2Cl2
Molecular weight: 84.93
Melting point: -970 C
Boiling point: 39.8-400 C
Water solubility at 200 C: 20 g/L
Hazards: Harmful, toxic, highly flammable, carcinogenic, and corrosive -
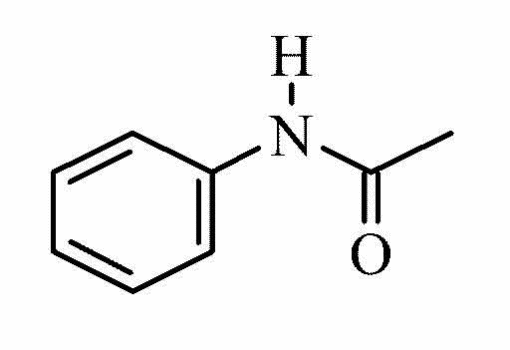
Molecular structure Acetanilide4
Form: Grey or White powder
Molecular formula: C8H9NO
Molecular weight: 135.16
Melting point: 113-1150 C
Boiling point: 3040 C
Water solubility at 200 C: 5 g/L
Hazard: Harmful, toxic, and irritant -

Molecular structure Sodium Sulfate5
Form: White crystals or powder
Molecular formula: Na2O4S
Molecular weight: 142.02
Melting point: 8840 C
Boiling point: 17000 C
Water solubility at 200 C: 18.5 mg/L -

Molecular structure Hazard: Irritant
Sodium Bicarbonate6
Form: White crystals or powder
Molecular formula: CHNaO3
Molecular weight: 84.01
Melting point: 3000 C
Boiling point: 8510 C
Water solubility at 200 C: 90 g/L
Hazards: Harmful and irritant -
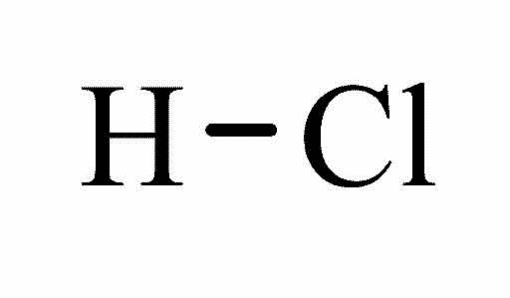
Molecular structure Hydrochloric acid7
Form: Fuming liquid
Molecular formula: ClH
Molecular weight: 36.46
Melting point: -350 C
Boiling point: 570 C
Water solubility at 200 C: Miscible
Hazards: Extremely flammable, harmful, irritant, toxic, and corrosive
Experiment Procedure
The experiment was initiated with a sample of Phensuprin exactly weighing 2 grams, which was added into a 125 ml Erlenmeyer flask. The next procedure involved addition of 50 ml of dichloromethane to the Phensuprin sample in the 125 ml Erlenmeyer flask. The content was then stirred rapidly until all soluble contents dissolved. The stirring process was rapid to ensure that all the solid particles of the Phensuprin sample were cracked and dissolved in dichloromethane. A filtering paper was used to gauge the amount of solid particles.
The filter paper was first weighed and its mass recorded in the laboratory data sheet for later experimental processes. This pre-weighed paper was required for the filtration of the content using a gravity filtration technique to separate the solid elements from the solution. The remaining solid was then gathered in the filter. This solid content was sucrose, and it was rinsed with 5 ml of dichloromethane to eliminate particles of other constituents of the Phensuprin.
The collected sucrose was then left in a beaker for one week to dry at room temperature. It was observed that after one week, the sucrose and the filter paper had completely dried and they were subsequently weighed to gauge the mass of sucrose contained in the 2 g of Phensuprin sample. The subsequent process involved determining the weight of the sucrose and its percentage composition, which were recorded in the laboratory data sheet.
An extraction process was applied to separate the mixture of the filtrate containing acetylsalicylic acid and acetanilide. Acetylsalicylic acid, the dichloromethane solution, was placed in a separating funnel, and it was separated through two processes by adding two fractions 5% sodium carbonate solution. The first portion of the 5% of the sodium carbonate solution measured at 25 ml was added to the solution of dichloromethane in the separating funnel. The separating funnel was then lightly shaken for about one to two minutes, and the pressure was released by opening the cork safety before the funnel could be put in a clam for subsequent separation of the contents of two layers. The bottom layer with dichloromethane was drained and trapped in 125 ml Erlenmeyer flask. The remaining layer with the aqueous compounds was transferred from the funnel into a beaker of 400 ml. The secluded dichloromethane contained in the Erlenmeyer flask was then transferred into the separating funnel to allow for the second extraction by applying the second fraction of 25 ml of 5% sodium carbonate. Further, the two aqueous were later mixed for subsequent separation while the dichloromethane was stored after extraction.
Ice cubes were used to cool the combined aqueous solution, and 10 ml of concentrated hydrochloric acid was poured gradually by transfer pipette as the content was stirred simultaneously. When the acid was transferred, the beaker was whirled intermittently because of the foaming that took place. Once all the hydrochloric acid was completely transferred into the beaker, the pH of the solution was gauged by using a pH paper using the drops obtained by a glass rod. The glass rod was used throughout the process to collect drops of the solution from the beaker to the testing pH paper until the preferred pH of less than two was achieved. Ice cubes were further used to cool the aqueous solution so that acetylsalicylic acid could be obtained in its solid state. Further, the procedure also involved the use of vacuum filtration to allow the collection of the solid formed. It was then rinsed with 5 ml of cold water. Prior to the filtration process, a vacuum pump was used for several minutes to ensure that the collected solid was dry. The collected acetylsalicylic acid solid was then allowed to dry for one week at room temperature in an open sample glass bottle. The solid had dried after one week, and it was weighed and mass recorded, as well as its percent composition.
The next procedure in the experiment involved the extraction of acetanilide. Anhydrous sodium sulfate was used to dry the dichloromethane solution. Anhydrous sodium sulfate was put on the dichloromethane solution as the content was swirled to obtain a good mixture. Sodium sulfate was slowly added in small quantities until it could clump and the excess sodium sulfate could freely flow in the mixture. A pre-weighed round bottom flask of 100 ml was used to collect the swirled and filtered solution from the mixture. Methylene chloride was the solvent obtain, and it was then evaporated by a rotary evaporator to ensure that acetanilide part of the mixture could be collected in a solid state. A round bottom flask was used to collect the solid, which was measured, mass recorded and the percentage composition determined and recorded in the laboratory data sheet.
The melting points of the three separated compounds, including sucrose, acetylsalicylic acid and acetanilide, were used to determine their purity. The results were compared against melting points presented in the literature.
Results
Calculation of percent composition of the three compounds
Mass of the Phensuprin: 2.0 g
Mass of the filter paper used to separate the sucrose: 0.95 g
Mass of the filter paper+ sucrose: 1.47 g
Mass of sucrose obtained: 0.52 g
Mass of acetanilide obtained: 0.4 g
Mass of the round bottom flask: 39.5 g
Mass of acetylsalicylic obtained: 0.5 g
Total mass: 0.52 + 0.4 + 0.5 = 1.42 g
Total percent recover: [1.42 g/2.0] x 100 = 71%
Percent composition of sucrose: [0.52/1.42] x 100 = 36.6%
Percent composition of acetanilide: [0.4/1.42] x 100 = 28.1%
Percent composition of acetylsalicylic: [0.5/1.42] x 100 = 35.2%
Synopsis of Results
The experiment was successfully performed, and all the three compounds found in Phensuprin were extracted. The two grams of the sample produced about 1.42 g of the three compounds, which represented about 71% extraction rate. About 0.58 g (29%) of Phensuprin contents was not recovered perhaps due to experimental errors in the extraction processes. The masses of acetylsalicylic acid, sucrose, and acetanilide separated were 0.5 g, 0.52 g, and 0.4 g respectively. Sucrose had the highest percentage of recovery at 36.6%, followed by acetylsalicylic acid at 35.2%, and finally acetanilide at 28.1% based on the total mass extracted from the sample of Phensuprin used in the experiment. The melting point of sucrose was 186.2 °C, which confirmed that it was a pure substance because literature also presents a similar melting point for the compound. However, both acetylsalicylic acid and acetanilide had lower melting points relative to ones found in the literature. The melting point of acetylsalicylic acid was 123 °C compared to 134-136 °C found in the texts, while acetanilide had a melting point of 96 °C compared to 113-115 0C indicated in the literature.
Discussion
Most organic reaction substances and other commercial products are compounds with different substances. Pure constituent substances of each element can be obtained through extraction of the mixture by relying variations in their physical and chemical properties.8 For instance, compound solubility properties in organic and inorganic solvents are vital for this extraction experiment. These variations in chemical and physical properties of substances have been used to extract individual constituents from samples.
The aim of this experiment was to extract various three components of Phensuprin using acid/base separation experiment. During the experiment, both solid and liquid products were obtained as defined by solubility of substances in organic and inorganic solvents. The three compounds found in Phensuprin, which is an analgesic, are sucrose, acetylsalicylic acid, and acetanilide. The initial process involved the separation of sucrose. Sucrose is soluble in water, an inorganic solvent, but acetylsalicylic acid and acetanilide could only dissolve in an organic solvent. Hence, a dichloromethane, an inorganic solvent, was used to dissolve acetylsalicylic acid and acetanilide, leaving sucrose as the only insoluble solid substance in the mixture.
Sucrose does not dissolve in a non-polar organic solvent, dichloromethane because it cannot create hydrogen bonds with the dichloromethane.
It was noted that sucrose could not dissolve in dichloromethane because it cannot form hydrogen bonds with the reactant. Hence, the gravity filtration was used to separate it from the solution. It is noted that filtration is a physical process that is used to separate solid particles from a liquid content by sieving or applying a membrane that only allows liquid to pass through while limiting soli particles. Sucrose particles were separated from dichloromethane solution using a filter paper because the filter paper only allowed the liquid to pass but not solid particles.
The filtered sucrose was further cleaned by washing using dichloromethane to eliminate traces of acetanilide and acetylsalicylic acid, which could have possibly made the particles impure. Cleaning is a critical process after filtration that eliminates all impure traces that could be present in collected residues. Hence, traces of acetylsalicylic acid and acetanilide would still be present in the sucrose without washing.
The reaction between acid and base was used to control the solubility of acetylsalicylic acid and acetanilide. While both acetylsalicylic acid and acetanilide are soluble in dichloromethane, sodium carbonate is used to alter the solubility of acetylsalicylic acid. Sodium carbonate reacted with acetylsalicylic acid to form sodium acetylsalicylate. In this reaction, sodium bicarbonate was used to provide the basic condition while the acetylsalicylic acid created acidity for the reaction to occur. Acetanilide does not react with sodium carbonate because of its low strength as a basic substance. Thus, acetanilide remained in the solution of dichloromethane. Additional of 25 ml drops of sodium carbonate was required to change all acetylsalicylic acid to sodium acetylsalicylate – a soluble salt. This reaction results in the creation of immiscible solutions of dichloromethane solution and sodium acetylsalicylate solution, which are organic and inorganic solutions respectively. The separating funnel is effective for separating two immiscible solutions due to its accuracy and efficacy. These solutions also have different density. As such, dichloromethane solution settled at the bottom while sodium acetylsalicylate solution formed the upper layer. The solution containing acetanilide was first drained and 25 ml of 5% sodium carbonate was added to remove all the retained acetylsalicylic acid.
Hydrochloric acid was used to extract acetylsalicylic acid from the aqueous solution. Hydrochloric acid was used to extract acetylsalicylic in a solid state, which was insoluble in water. The reaction involved hydrochloric acid, sodium elements, and acetylsalicylate to produce acetylsalicylic. Acetylsalicylic was precipitated using ice from the solution and then separated using filtration. The pH of 2 was ideal for sodium acetylsalicylate re-protonation, leading to its precipitation in the ice. Sodium carbonate used specifically to reduce the pH of the solution and make it stable.
Acetanilide was separated from the dichloromethane solution using anhydrous sodium sulfate. Anhydrous sodium sulfate was used a drying agent in order to get dry acetanilide. Anhydrous sodium sulfate has ability to absorb water from other substances. Dichloromethane was separated from acetanilide solution using a rotary evaporator because it was an inorganic solvent.
This extraction experiment was progressive based on solubility of the three compounds found in the sample. Sucrose was initially extracted using gravity filtration, which depends on gravity for particle filtration. On the contrary, vacuum filtration was preferred for acetylsalicylic acid separation because it is faster, acts as a drying agent, and can tolerate high boiling points. It was faster relative to gravity filtration because of vacuum pressure applied in the pressure.
Different masses of the three compounds were obtained. Sucrose was 0.52 g, acetylsalicylic acid was 0.5 g while acetanilide was 0.4 g. The percentages of extracted sucrose, acetylsalicylic acid, and acetanilide were 36.6%, 35.2%, and 28.1% respectively. These percentages represented the most abundant component of Phensuprin. Sucrose was the most abundant, followed by acetylsalicylic acid, and finally acetanilide of the recovered Phensuprin. The total mass of the separated components was 1.42 g from the original sample of 2.0 g, representing 71% recovery rate of Phesuprin.
The pure form of sucrose was recovered because it had a similar melting point as indicated in literature. On the contrary, it was noted that the melting point of acetylsalicylic acid was 1230 C while acetanilide was 960 C. These melting points were lower than the melting points indicated in literature. Hence, these two substances contained traces impurities. Impurities lower melting points of substances. Unfortunately, the presence of impurities showed chemical extraction was not always completely efficient. As such, it was nearly impossible to separate all original substances contained in Phensuprin as pure single components. In addition, each constituent of Phensuprin was most likely to have traces of one or more of the extracted compounds. To extract pure compounds, recrystalization was needed by following recommended laboratory procedures.
Notably, experimental processes could have led to some errors. For instance, sucrose was washed, aqueous solutions were separated using various reactants and stored for one week. Ineffective processes could have contributed to errors and presence of impurities in other extracted substances. The pH of the aqueous solution was also controlled to ensure that acetylsalicylic acid could be obtained. However, improper processes could have led to poor solubility of the contents.
References
Lide, David R. CRC Handbook of Chemistry and Physics. 79th ed. 1999. Boca Raton, FL: CRC Press, Inc,. Print.
National Center for Biotechnology Information. Compound Summary – Acetanilide. 2016. Web.
—. Compound Summary – Acetylsalicylic Acid. 2016. Web.
—. Compound Summary – Hydrochloric Acid. 2016. Web.
—. Compound Summary – Sodium Bicarbonate. 2016. Web.
—. Compound Summary – Sodium sulfate. 2016. Web.
—. Compound Summary – Sucrose. 2016. Web.
O’Neil, Maryadele J. The Merck Index – An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th ed. 2006. Whitehouse Station, NJ: Merck and Co., Inc,. Print.
Zubrick, James W. The Organic Chem Lab Survival Manual. 5th ed. 2001. New York: John Wiley & Sons, Inc,. Print.
Questions
- The original organic solution was extracted two times with aqueous sodium bicarbonate. After the extraction, what compound(s) were in the organic layer? What compound(s) were in the aqueous layer?
The organic layer had dichloromethane and acetanilide while the aqueous layer had acetylsalicylic acid. - Assume that both acetylsalicylic acid and acetanilide are soluble in diethyl ether, and that diethyl ether was used in place of the methylene chloride. Would the ether layer be the bottom layer in the separatory funnel or the top layer? Explain your answer.
The density of water, which is 1.0 g/mL, is comparatively higher than the density of diethyl ether, 0.71 g/mL and, thus, diethyl ether can be found on the top layer. - Assume that both acetylsalicylic acid and acetanilide are soluble in methanol, and that methanol was used in place of the methylene chloride. What problem(s) might occur during the extractions? (Careful, this is a trick question.)
The solubility challenge will occur since the solution would be homogenous. Methanol, a polar solvent, is not considered as an extraction solvent in this case because it is miscible with water and, thus, will not form a different layer. Instead, the resultant mixture will be a homogenous mixture of acetylsalicylic acid and acetanilide. Thus, no formation of layers would occur to enhance the separation of acetylsalicylic acid and acetanilide. - Historically, the solid residue that is left after the methylene chloride solution is evaporated has a lower melting point that its literature value, due to impurities. Explain what impurities are present and why.
Used substances, such as sodium carbonate, acetylsalicylic acid, and anhydrous sodium sulfate, are most likely impurities that can affect melting. These impurities are observed, as shown by low boiling points, because the separation process is not absolutely perfect and, therefore, traces of substances used or contained in the samples and reactants can be detected.
Footnotes
- National Center for Biotechnology Information. Sucrose. 2016. Web.
- National Center for Biotechnology Information, Acetylsalicylic Acid. 2016. Web.
- O’Neil, Maryadele J, The Merck Index – An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed, Whitehouse Station, NJ: Merck and Co., Inc, 2006, p. 140.
- National Center for Biotechnology Information, Acetanilide. 2016. Web.
- National Center for Biotechnology Information.Sodium Sulfate. 2016. Web.
- National Center for Biotechnology Information.Sodium Bicarbonate. 2016. Web.
- National Center for Biotechnology Information.Hydrochloric Acid. 2016. Web.
- Zubrick, James W, The Organic Chem Lab Survival Manual. 5th ed. 2001. New York: John Wiley & Sons, Inc,. p 110-122.
