Introduction
A seed may be defined as the flowering plant’s unit of reproduction, which can develop into a new plant. Therefore, seeds ensure the continuity of plant reproduction in plants that produce them. Most seeds exist in a dry form that makes them capable of staying dormant for prolonged periods. Such seeds are viable and can only bring rise to new plants through the process of germination. Germination of seeds requires the availability of three main substances: water, oxygen, and warmth (Rajjou et al., 2012). The germination of certain plants is dependent on the availability of light or darkness. During germination, mature seeds take in large amounts of water to trigger cellular metabolism and growth. Seeds take water through a process known as imbibition, which causes the seeds to puff up and disrupt the seed coat. Imbibition activates hydrolytic enzymes that degrade the food resources stored in the cotyledon into metabolically useful chemical substances (Tan, Chen, Wang, & Dai, 2013). The radicle of the seed grows into roots, which push down into the soil to absorb water and minerals. Conversely, the plumule grows upwards into the shoots that later become leaves. Germination depletes the stored nutrients necessitating the leaves of the seedling to synthesize food when sunlight is available in a process called photosynthesis. The seedling ultimately grows to become a new plant.
The processes of diffusion and osmosis are also crucial to the germination and development of plants. Diffusion, which is the net transfer of molecules from an area of high concentration to low concentration helps in the uptake of oxygen and carbon dioxide from the air into the plants through the stomata. Water and ions of contained in inorganic salts get into plants from the soil through their root cells by diffusion. The amount of diffusion is determined by dynamics such as temperature, concentration gradient and the size of diffusing particles.
Plants depend on the nutrients and water in the soil to survive. For water uptake to occur, plant cells experience a phenomenon called osmosis. Osmosis has a crucial role in the survival of plant life. By definition, osmosis is the spontaneous movement of a solvent (water) through a cell membrane. It is a unique type of diffusion that transports water molecules from a region with a higher concentration to a region with a lower concentration to stabilize the cellular environment. Osmosis goes on until the pressure of the fluid on both sides of the semipermeable membrane is equal. Osmosis ensures the turgidity of plant cells, which provides support to plants. Osmotic pressure also opens the stomata in the leaves thus allowing plants to take in carbon dioxide from the air for photosynthesis.
The aim of this experiment was to determine the effect of varying concentrations of calcium chloride on the germination of seeds. It was hypothesized that increasing the concentration of calcium chloride would reduce the rate of germination.
Methods
A germination experiment was done to determine the effect of different salt concentrations on the germination of seeds. Different solutions of CaCl2 (0.0625M, 0.125M, 0.25M and 0.5M) were prepared. Ten seeds were then placed over paper towels made wet with the different concentrations of CaCl2. The paper towels and the seeds were then placed in well-labelled individual Ziploc bags and sealed. The treatments were replicated 4, 6, 5, and 6 times for the 0.0625M, 0.125M, 0.25M and 0.5M concentrations respectively. For each replicate, a control containing distilled water instead of the CaCl2 solution was used. The changes on the seeds were observed on a daily basis for seven days and recorded. The null hypothesis was that the average germination on day 7 will not be different among groups. The null hypothesis was to be rejected if p-values were equal to or greater than 0.05.
Results
All seeds soaked in distilled water germinated by the end of day 7. The rate of germination increased steadily by the end of day 7 for the all CaCl2 concentrations. However, the highest rate of germination was observed in the 0.062M CaCl2 concentration while the lowest germination was seen in the 0.5M CaCl2 groups. The overall trend was that the germination rate of germination decreased with an increase in the concentration of CaCl2. The findings are summarized in Figure 1.
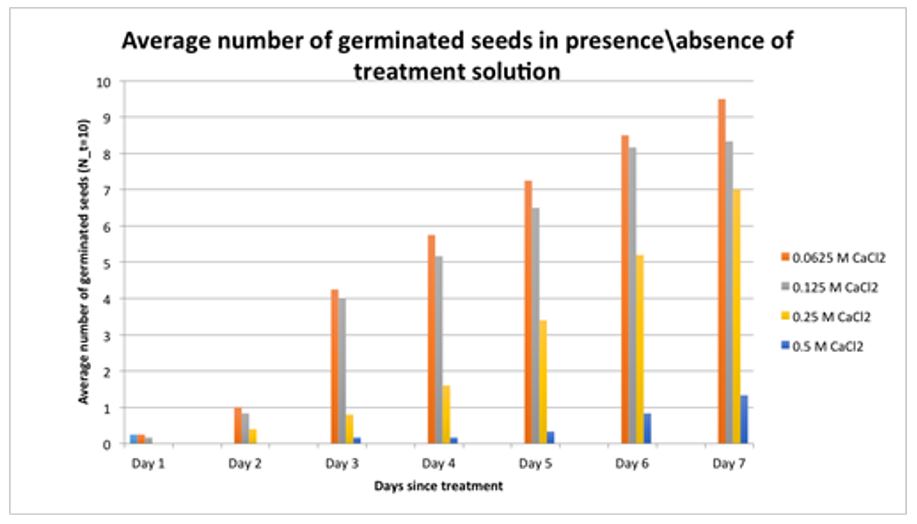
The findings of the study rejected the null hypothesis. It was observed that the average germination on day 7 between the 0.0625M groups and 0.5M groups were statistically significant (p=0.0395). Additionally, the average germination between the control and the 0.125M, 0.25M, and 0.5M CaCl2 groups were statistically significant at p-values of 0.0297, 0.0256 and 0.0000 respectively. The findings are summarized in Table 1.
Table 1: Pairwise comparison of the differences in germination between treatment groups.
P-values indicated with an asterix are statistically significant.
Discussion
The objective of the study was to determine the effect of different concentrations of CaCl2 on the germination of seeds. It was hypothesized that there would be no differences in the germination rates in different groups by the end of day 7. However, the null hypothesis was rejected because it was observed that there were differences between the rate of germination in 0.0625M and 0.5M CaCl2. Also, there were significant differences in the germination rates between the controls and increasing concentrations of CaCl2. These findings could be attributed to the impact of CaCl2 on the process of imbibition, which is the first step in germination. Imbibition is a special form of diffusion where the solid particles of a substance take in large amounts of water without the formation of a solution. Increasing the concentration of water by the addition of CaCl2 reduces the rate of imbibition since it reduces the concentration of water available thus reducing the concentration gradient and the flow of water into the seeds. The findings of this study corroborated those reported by Mirmazloum, Szabo, PoorKalhor, and Németh (2010) where an increase in CaCl2 concentration reduced the germination rate of Melissa officinalis L. and Ocimum basilicum L.
The observed reduction in germination in the presence of salts is the consequence of the combined effect of osmotic and ionic factors (Panuccio, Jacobsen, Akhtar, & Muscolo, 2014). Increasing the concentration of CaCl2 raises the number of ions present in the roots and shoots of the germinating seed thus causing ion toxicity. Toxicity occurs when salt overload surpasses the capacity of cells to compartmentalize salts in the vacuole leading to an accumulation of salts in the cytoplasm and the inhibition of enzyme activity. The salts may also build up in the cell walls and dehydrate the cell leading to reduced germination. The experiment could be improved by using equal replicates for each treatment solution.
It was concluded that water is an essential component for the germination of seeds. The addition of CaCl2 to seeds decreases the rate of germination by exerting ion toxicity and reducing the osmotic pressure required for the intake of water. The harmful effects of and CaCl2 increase with an increase in the concentration of the salt.
References
Mirmazloum, S.I., Szabo, K., PoorKalhor, V. & Németh, É. (2010). Effects of different levels of NaCl and CaCl2 on seed germination characteristics of Melissa officinalis L. and Ocimum basilicum L. International Journal of Horticultural Science, 16(5), 21–25.
Panuccio, M. R., Jacobsen, S. E., Akhtar, S. S., & Muscolo, A. (2014). Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants, 6 (plu047), 1-18.
Rajjou, L., Duval, M., Gallardo, K., Catusse, J., Bally, J., Job, C., & Job, D. (2012). Seed germination and vigor. Annual Review of Plant Biology, 63, 507-533.
Tan, L., Chen, S., Wang, T., & Dai, S. (2013). Proteomic insights into seed germination in response to environmental factors. Proteomics, 13(12-13), 1850-1870.
