Introduction
The increasing depletion of world resources coupled with the devastating effects of population is rapidly calling for the need to devise cleaner and renewable sources of energy (North Atlantic Treaty Organization, 1995). Of particular concern are the automobiles. Their pollution rate is alarmingly high besides the escalating costs of oil resources. Hence, it has become necessary to seek alternative energy sources that are compatible with the changing times and civilization. As a result, the use of hydrogen is being considered as a better alternative. In any case, vehicles that are powered by hydrogen operate in the same way just like those which use gasoline. The only difference is the modality used to power the engine.
The electrochemical reaction between hydrogen and oxygen generates electric energy that is used to power the engine. The only by-product emitted into the atmosphere is water in form of vapour. Hence, the much dreaded carbon dioxide is not emitted as compared to the use of gasoline. This paper elaborates on the merits of hydrogen as an alternative source of energy and how it powers automobiles. To begin with, some of the properties of hydrogen as a fuel are discussed with special emphasis on how it compares to other fuels. Additionally, the working principle of hydrogen-driven automobiles is highlighted and finally, a critical literature review of hydrogen as a fuel concludes the paper.
Properties of Hydrogen
Hydrogen has immense benefits compared to other non-renewable fuels in its function as a transportation fuel. Firstly, it is vital to note that hydrogen is one of the most abundant elements in the atmosphere and therefore it cannot be depleted any time soon. In addition, the element offers the chance to use a clean source of energy that does not pollute the environment (DeGunther, 2009).
As a gas, hydrogen undergoes the process of combustion so that it can provide the much needed electric energy to power motor engines. To attain a higher level of combustion, the gas has a low density, high rate of diffusion, highly flammable as well as the ability to ignite itself at a relatively lower temperature. Moreover, hydrogen has a rapid flame velocity that enables it to burn faster than ordinary fossil fuels. These properties are indeed needed for an internal combustion engine of a car that has been designed to use hydrogen.
Hydrogen Fuel Vehicles
The use of hydrogen as an alternative source of fuel has been applied in the running of two different types of automobiles. The first type is referred to as the Fuel Cell Vehicle (FCV). Although there are still myriad of challenges that must be overcome in order to fully develop this type of vehicle, the success level is already above par. This type of hydrogen vehicle works through the conversion of internally stored chemical energy to mechanical energy that initiates motion (Hollinger & Tapan, 2008). On the hand, the hydrogen international combustion engine is almost similar to a gasoline engine. However, it has been cushioned with slight modifications in order to meet the safety and efficiency requirements of the engine.
Hydrogen internal combustion engine
As already mentioned, a hydrogen internal combustion engine (ICE) car uses a conventional ICE but whose working principle is slightly different from that which uses gasoline. Hydrogen powered engines are mostly beneficial due to their abilty to operate singly on hydrogen or with a mixture of hydrogen and natural gas that has been compressed (Bose & Malbrunot, 2007). Firstly, the injector receives hydrogen that has been stored in a container and pressured through a regulator. In order to start the combustion process of hydrogen, plenty of oxygen is required. Hence, this efficiency level is achieved by the use of a turbo charger at a stable temperature of about 60 degrees. This is necessary to avoid any eventuality of an explosion. The system is also fitted with a coolant that lowers the temperature of the turbo charger. The figure below illustrates the working principle of a hydrogen internal combustion engine (Bose & Malbrunot, 2007)
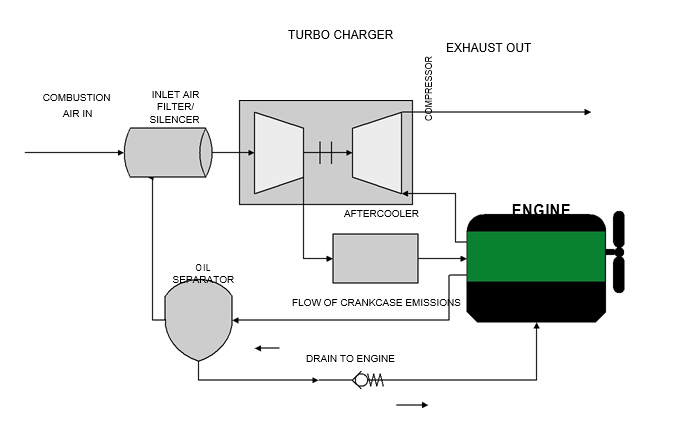
Hydrogen fuel cell
A hydrogen fuel cell is deemed to be more effective than the hydrogen powered internal combustion engine. The overall process maps into the creation of electric current occasioned by the conversion of energy from the electrochemical to mechanical. Both hydrogen and oxygen are reacted together in the presence of a catalyst in both the positive (anode) and negative (cathode) electrodes (Thomas, C.E. et al., 2000). Reduction takes place at the negatively charged electrode where oxygen undergoes reduction by gaining electrons. At the anode, hydrogen is oxidized by losing electrons. Thereafter, the swapping of electrons that takes place in the electrolyte leads to the generation f electric current needed to force the movement of the vehicle. The figure below illustrates both the cathode and anode arrangement of this cell as well as the movement of electrons through the electrolyte.
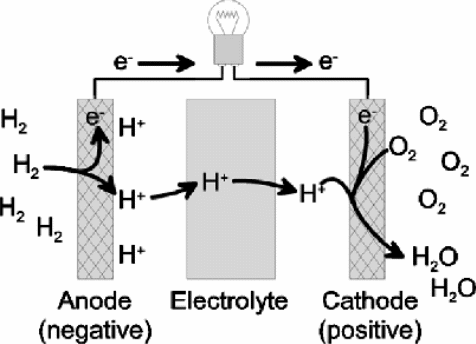
Challenges
Hydrogen has a much higher volume that that of gasoline in spite of its low density (Birch, 2007). As a result, the driving range for a hydrogen powered vehicle is much shorter compared to a gasoline automobile. In addition, the use of hydrogen as a fuel is quite costly bearing in mind that its production process is equally lengthy and demands more time and financial resources.
Conclusion
In spite of the limitations posed by the use of hydrogen in automobiles, it is imperative to note that this form of energy has a brighter promise for the future due to its clean nature and renewability. As fossil fuel deposits continue to deplete and wreck havoc to our environment through the emission of greenhouse gases, hydrogen remains to be the better option for global energy sustainability.
References
Birch, S. (2007). Hydrogen, the IC engine, and the future. Automotive Engineering International, 115(6), 28-29.
Bose, T & Malbrunot, P. (2007). Hydrogen: Facing the Energy Challenges of the 21st Century. London: John Libbey Eurotext.
DeGunther, R. (2009). Alternative Energy for Dummies. New Jersey: Wiley Publishing Inc.
Hollinger. T & Tapan. B. (2008). Hydrogen technology mobile and portable application, A.Leon (ed.), Hydrogen technology, -Verlag Berlin Heidelberg: Springer.
North Atlantic Treaty Organization (1995). Hydrogen energy system: production and utilization of hydrogen and future aspects Scientific Affairs Division. London: Kluwer Academic Publishers.
Thomas, C.E. et al. (2000). Fuel options for the Fuel cell vehicle: hydrogen, methanol or gasoline? International Journal of Hydrogen energy. 25: 551-567
