Abstract
Basing on several studies, it can be noted that removal of organic components from wastewater is based on several factors like solution’s chemistry, physiocochemical properties and membrane characteristics. The main objective of this study was to investigate different aspects related to nano-filtration in the laboratory such as reactive dye desalting and concentration. It was observed that NF270 is more efficient in removing organic molecules from the solution than the NF270. The study also demonstrated that nano-filtration is very important in dye desalting and concentration.
Introduction
The wastewater for textile industry consists of a large proportion of chemicals and dyes (Kant 2012). It is known that these chemicals and dyes are reactive and non biodegradable, hence, they can be harmful when discharged to the environment. Also, textile industries use a lot of water in the production of fabric (Şen & Demirer 2003). Therefore, this wastewater before it’s reused; it needs to be treated in order to remove these reactive elements and dyes. There are various processes that could be used to treat this wastewater and they include biological or physical processes (Valh et al. 2011).However, there are several disadvantages and advantages of treatment technology. The disadvantages include complexity of the processes due to varying dyes in the wastewater after an hour or days (Türgay et al. 20110). There is as well a huge amount of solid suspension in the wastewater and this makes it complicated to treat wastewater from textile industry (Kim et al. 2000). Hence, it becomes more costly and less efficient. The dyeing and printing sections are known to produce wastewater that is of different types in textile plant (Tang & Chen 2002). This wastewater is full of chemicals, reactive dyes and there is need for treatment of this wastewater before being discharged to the environment or before being reused (Libra & Sosath 2003).
Literature review
The literature review majorly focuses on different aspects of nano-filtration process which is known to be very critical in dye desalting and concentrating (Sahinkaya et al. 2008).
The Physiocochemical and physical treatment technique
This method aims at removing undissolved chemicals and particulate matter from the wastewater. The technique involves flocculation, irradiation, membrane technology adsorption, coagulation, ion exchange (Lin & Chen 1997). But the major focus of the current study is on membrane technology which is considered to be the best alternative of these other processes because it’s a process termed as commercially feasible as well as technically critical in the textile wastewater treatment (Fu & Wang 2011). There are several studies undertaken on ultra-filtration, microfiltration and nano-filtrations. Micro-filtration is effective in colloidal dye removal from dye bath. Ultra-filtration is suitable in the treatment of wastewater of a single step (Ben Amar et al.2009). For nano-filtration, organic molecules as well as salts of low molecular weights can be separated (Marcucci et al. 2002). The advantage with nano-filtration process is that it has the capacity of using pressure that is moderate as well as it is able to recover water of about 99% from the wastewater (Jakobs & Baumgarten 2002). This water is seen to be adequate enough in terms of quality to be recycled to the process of dyeing. The salinity is observed to be low for the nano- filtration permeates (Valh et al. 2011).
Membrane Technology
By definition, semi-permeable membrane is considered to be a thin layer element which has the capacity of separating a given component as a result of pressure difference between the permeate and the feed across the membrane (ArumaiDhas 200). Semi-permeable membrane can also be defined as a selective barrier that has the capacity of allowing particular dissolved materials and particles to pass through as well as retain the other materials hence not passing through the membrane (Tzitzi et al. 1994). The selection of this material by the membrane is based on the molecules charge, size of the molecules, shape membrane pore size and applied pressure (Verma et al. 2012). These membranes can be classified into three groups that include nano-filtration, micro-filtration and reverse osmosis (Van der Bruggen & Vandecasteele 2003).
Nano filtration
The advantage with nano-filtration process is that it has the capacity of using pressure that is moderate as well as it is able to recover water of about 99% from the wastewater (Wang et al. Z 2011). This water is seen to be adequate enough in terms of quality to be recycled to the process of dyeing (Gozálvez et al. 2008) Studies have been undertaken to demonstrate the potential of nano-filtration in the dye solutions of industries treatment, pure dyes solutions treatment as well as effluent from textile dye plants (Hong et al. 2006).There are as well other studies that demonstrated aqueous dye concentrating and desalting at a dye generating plant. These demonstrations indicated that NF is more efficient, less costly and the water produced was of high quality. Koyuncu (2002) demonstrated that when wet oxidation and nano-filtration processes are combined in the treatment of wastewater, there was at-most 97 percent COD as well as 99 percent colour removal (Rautenbach & Groeschl 1990). Conventional nano-filtration needs pre-treatment with chemicals that are of high quantity in order to eliminate the natural and synthetic oils that is known to foul the membranes and this leads to the wastages of chemicals and the removal of water (Schaep et al. 1998). Membranes that have low rejection for dissolved materials are considered to have a great significance in the technology of separation and reverse osmosis membrane was referred to as nano-filtration membrane(Conlon & McClellan 1989). Apart from dye concentration and ionic strength, aggregation is as well observed to increase due to introduction of alkyl compounds. This is because the alkyl chains increases the hydrophobic interaction. When organic solvents and ionic solubilising groups are introduced on the other hand, aggregation is observed to decrease (Gozálvez et al.2008). In regards to aggregation modification, it is known that the effect of hydrophobic currently never plays a big role.
Membrane Workability
Fouling
During nano-filtration, there is occurrence of a condition referred to as concentration polarization(CP).This usually happens in cases where there is rejection of the solutes by the membrane and in this case, the processes of nano-filtration involves transportation of components as well as water through the membrane (Lau et al. 2009). In this case, there is retention of larger components in the solution while there is filtration of the smaller solutes or entities through the membrane. The larger size components are retained because there sizes are bigger than that of the pores while the smaller ones go through the membrane because of their small size which is smaller than the membrane pore (van der Graaf & Roorda 2000). There is always a reverse diffusion at the level where the retained entities accumulation concentration attains a level that is higher than the interface and the bulk surface of the stream (Australian Industry Group 2012). Basically, component accumulation that occurs at the interface is termed as concentration polarization (Jakobs & Baumgarten 2002).
By definition, fouling is the particulate or solute deposition on the surface or within the membrane. It is very hard to reverse this process and the flux change is lost over time including the solute rejection reduction as a result of concentration polarization (Joshi & Purwar 2004). There are higher expenses involved related to filtration operation as a result of both the flux decline and fouling related problems (van der Graaf & Roorda 2000). There are various components that are considered to be foulants and they include biological materials, suspended solutes, and inorganic scalants as well as organic matter (Lau & Ismail 2009). The occurrence of fouling is in a discrete phases that include hydrophobic adsorption, pore blocking and concentration polarization (Lau & Ismail 2009).
The phenomenon of pore blocking entails clogging of pores as a result of species being smaller than the pore sizes and the species are observed to be adsorbed onto the inner walls of the pores (Robinson et al. 2001). Also clogging can happen when there is no proper cleaning of the pores by the chemicals in order to eliminate the components adsorbed in the pores (Kabil & Ghazy 1994).There could be fouling that is irreversible due to ineffective concentration polarization (Lau & Ismail 2009). In order for the filtration process to be efficient in the system of NF, fouling control is considered to be very critical (Bes-Pia et al.2005). There has been an indication by previous studies that aggregation tends to be higher in dyes that are hydrolysed (Ciardelli & Marcucci). In this case, when there is higher ionic components in the dye solution, there will be less the tendency of aggregation (Judkins & Hornsby 1978).Furthermore, since the charged groups represent the ionic group number, there are five groups that are negatively charged for the hydrolysed concentration polarization reactive black (Hihara et al. 2000). But there are 4 groups for the un-hydrolysed one (Hamlin et al.1999). There were experiments undertaken on nano-filtration whereby there was placement of sodium chloride and Reactive Black 5 solution, hydrolysed in nature with the synthetic solution of dyes (Hamlin et al.1999). In this case, the aggregate number was expected to increase (Ciardelli & Ranieri 2001). Such could happen only if there was accumulation of the dye in the rejectant (Biolchi et al. 2006). This was not the case based on the result, because the aggregation number reduced to about half the original one (Hamlin et al.1999).There is a high affinity of dye ions in terms of self association. Self association can occur when these dye ions are mixed together with water. The dye ions form dimmers at a low concentration (10−4–10−6 M) (Gozálvez et al.2008).In this case, there is a combination of dye molecules whereby the process ends before other subsequent aggregation steps (Burkinshaw et al.2000). It is observed that the dimmer aggregation number is two. Dye ions have a high affinity to self-associate when they are mixed with water to form a solution. When the dye concentration is very low (that is between 10−4–10−6 M), the dyes exist as dimmers. In dimerization, the identical dye molecules combine and this process is complete before further aggregation can take place (Zaghbani 2007).
Methodology
Materials
In the experiment, there was application of chemical materials in order to achieve the intended goal. There was use of sodium chloride as an inorganic salt since it’s frequently used in textile industry for improving the dye of the fabric. The dye that was used in the experiment included reactive Remazol brilliant Blue dye. Distilled water was used as the solvent for both the dye and sodium chloride. There were two types of nano-filtration membrane used and they included DOW FILMTEC –NF245 and DOW FILMTEC –NF270.
Equipment
The experiment equipment included beakers, funnels, water stirrer and weighing balance connected to the computer for data collection. A stirred cell was used in the nano-filtration process and it had a capacity of 180 mL. UV spectrophotometer at 577nm wavelength was used in the determination of the dye’s solution absorbance. Also the conductivity meter as well as the pH meter was used to measure the conductivity and the pH of the solution respectively. Nanosizer was used measure the size of the particle.
Results
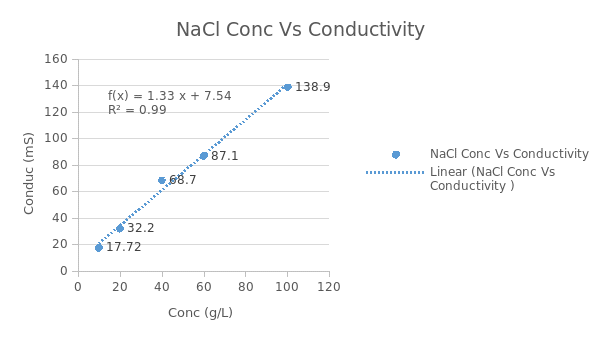
According to figure 3 above, it is clearly evident that conductivity is directly proportional to the concentration of sodium chloride in the feed during nano-filtration process. For instance, at 100g/l of sodium chloride the conductivity is high but at 20g/l the conductivity is observed to be low. This means that, when sodium chloride is salted out, the conductivity will decrease.
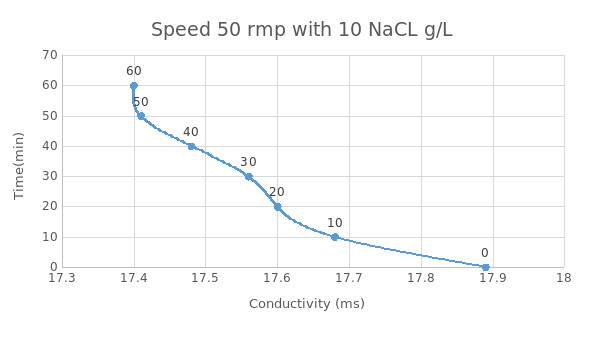
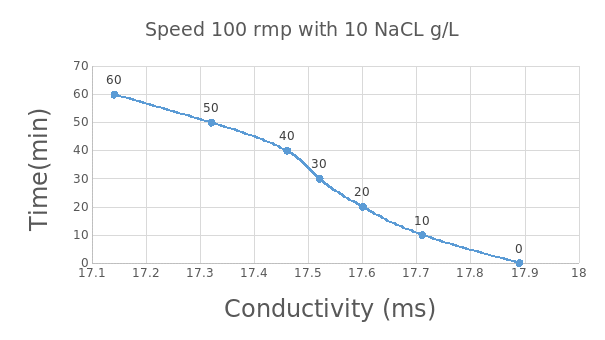
According to figure 4 and 5 above, at a speed of 50rmp and 100 rmp with 10NaClg/L, the conductivity is observed to decrease with increase in time. Salt rejection is seen to be 25% at 50rmp and 28 at 100rmp. For 50rmp, at time zero, the conductivity is observed to be 17.9 but it leveled at 17.4.This is similar at 100rmp but the difference is that the conductivity leveled at a lower level when compared with that at 50rpm. This therefore means that the speed is directly proportional to salt rejection. Also at high salt concentration and low string speed, the salt rejection is low.
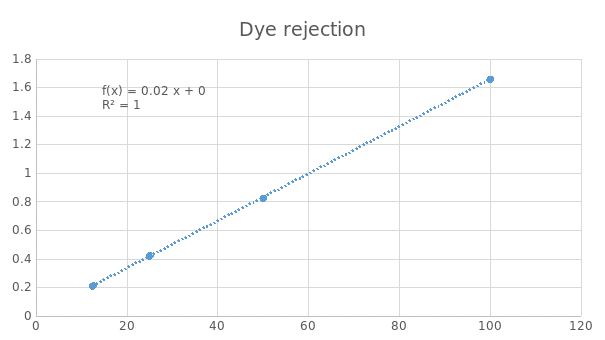
Figure 6 above shows absorbance against dye concentration. It is observed that at high dye concentration, the absorbance is high hence high level of dye rejection. This implies that dye concentration is directly proportional to absorbance and dye rejection is inversely proportional to absorbance.
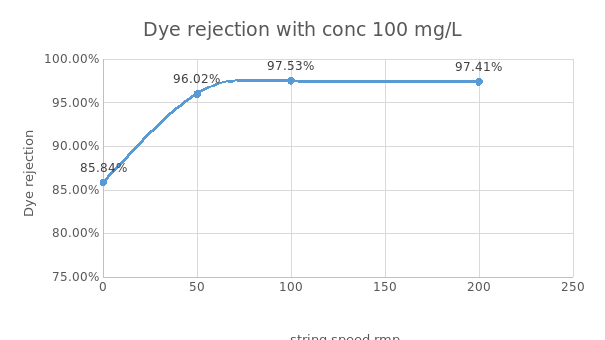
Basing on figure 6 above, it is observed that at high string speed and high dye concentration, the dye rejection is high. It is seen that the maximum dye rejection is 97.41%.This implies that dye rejection if directly proportional to string speed.
Table 1. Parameters of nano-filtration Process for NF245 and NF270
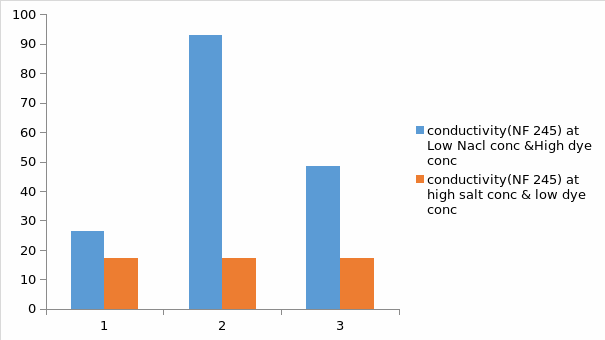
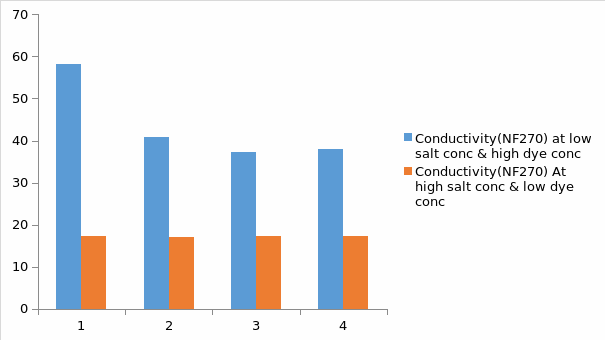
Basing on figure 8 and 9 above, it can be observed that salt concentration has an effect on the nano-filtration process. For figure 8(NF245), it is observed that at low salt concentration and high dye concentration in the feed, the conductivity was lower than that of when the salt concentration was higher and the dye concentration was lower after the process of nano-filtration. These observations are similar to the NF270, but the only difference is that for the NF270, the conductivity is higher than that of NF245. In this case, basing on the result it is clearly evident that NF270 is seen to partially soften the solution hence this character makes it appropriate for preserving the original quality of water. It can also be seen that NF270 is more efficient in removing organic molecules from the solution than the NF270.Due to high flux for the NF270, there is high yields of the products when compared with the NF245.Lastly, the quality and performance of NF270 is seen to be better than that of NF245
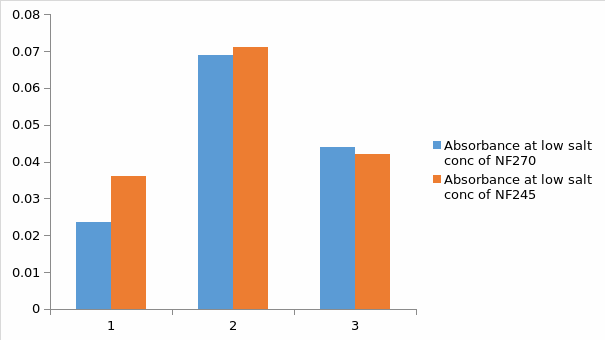
Basing on figure 10.It is clearly evident that the absorbance of both membranes is similar at low salt concentration and high dye concentration. On the other hand, absorbance was negligible at high salt concentration and low dye concentration. It can be concluded that the type of membrane has no effect on the absorbance.
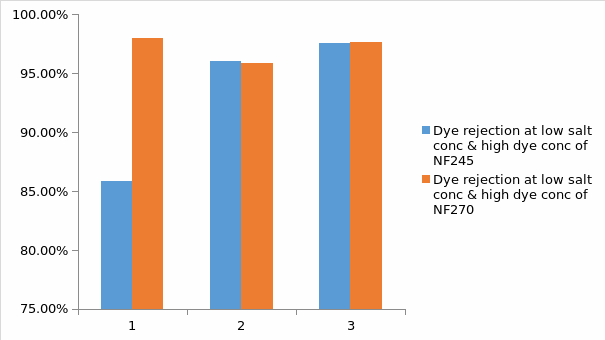
In regards to figure 11 above, it is observed that there is a small difference of dye rejection percentage between the two membranes. In this case, it implies that the type of membrane has less effect on dye rejection. It can also be noted that there is high percentage of dye rejection at high dye concentration and low salt concentration. This is because, at high dye concentration, there is high aggregation number. This is supported by the results whereby at low dye concentration and high salt concentration, the dye rejection percentage was not recorded.
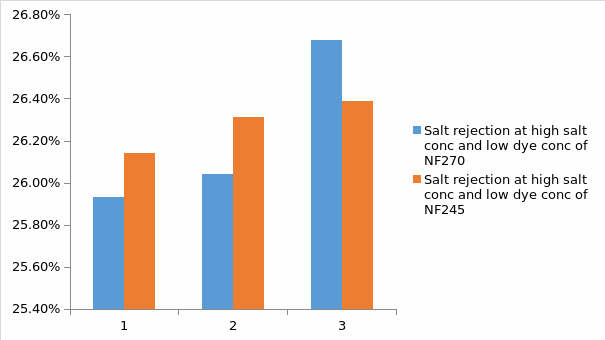
Basing on figure 12, it is observed that salt rejection is similar in both membranes, and it can be concluded that there is very little effect of membrane type on salt rejection. Also it can be noted that at high salt concentration and low dye concentration, salt rejection percentage is higher when compared with that of low salt concentration solution. In this case, basing on the results, it was observed that at low salts concentration; the salt rejection percentage was not recorded or was low. This is also similar to dye rejection whereby, the dye rejection percentage was not recorded or was low at low dye concentration but it was high at high dye concentration. All this observations are attributed to aggregation number of both sodium chloride and the dye basing the concentration of the dye and salt in the feed.
Discussion
Basing on several literatures, it can be noted that the removal of organic components from the wastewater is based on several factors like solution chemistry, physiocochemical properties and membrane characteristics. In relation to this, there is less information regarding the impacts of fouling on the organic component retention (Lau et al. 2009).The current study demonstrated that nano-filtration is very important in dye desalting and concentration. The dye rejection as well as salt rejection by the DOW FILMTEC membrane of nano-filtration was explored in this study. There was a decline in sodium chloride rejection with decrease in salt concentration. The rejection of the two dyes was above 90 percent at low salt concentration and high dye concentration. Similarly, this was the case with that of sodium chloride, whereby, there was a decline of salt rejection with decrease in salt concentration of the feed. A drastic dye aggregation number decrease is associated with the high sodium chloride concentration and this allows the formation of small aggregates (Zaghbani 2007). The removal of sodium chloride aggregate in the permeate never influences the dye aggregation as a result of the sodium chloride in the feed. All the experiment observations are attributed to the aggregation number of both the sodium chloride and the dye basing the concentration of the dye and salt in the feed (Gozálvez et al. 2008). This is supported by previous studies which noted that aggregation tends to be higher in dyes that are hydrolysed. In this case, when there are high ionic components in the dye solution, there will be less tendency of aggregation.
For figure 8, it is observed that at low salt concentration and high dye concentration in the feed, the conductivity will be lower than that of when the salt concentration is higher and the dye concentration is lower after the process of nano-filtration. The reason for this observation could be at low salt concentration, there will be high sodium chloride aggregation and this will lead to the removal of the ions from the feed hence low conductivity (Marrot & Roche 2002). But still, at high dye concentration the conductivity will be low since the dye is a poor conductor. These observations are similar to the NF270, but the only difference is that for the NF270, the conductivity is higher. The reason for this could be due to the differences in their characteristics. In this case, basing on the result it is clearly evident that NF270 is seen to partially soften the solution hence this character makes it appropriate for preserving the original quality of water. It can also be seen that NF270 is more efficient in removing organic molecules from the solution than the NF270.Due to high flux for the NF270; there is high yield of the products.
Conclusion
In conclusion, basing on several literatures, it can be noted that removal of organic components from wastewater is based on several factors like solution chemistry, physiocochemical properties and membrane characteristics. In relation to this, there is less information regarding the impacts of fouling on the organic component retention.The current study demonstrated that nano-filtration is very important in dye desalting and concentration. The experiment results indicated that nano-filtration process is very critical in concentrating as well as dye desalting.
References
ArumaiDhas, J 2008, ‘Removal of Cod and Colour from Textile Wastewater Using Limestone and Activated Carbon.’ Universiti Sains Malaysia, vol.143, no.3, pp. 243-253.
Australian Industry Group, 2012, ‘Water saving factsheet: Textile Industry’, Australian Industry Group, vol. 68, no. 3, pp. 748-953.
Ben Amar , N, Kechaou N , Palmeri , J , Deratani A , Sghaier ,A 2009,’Comparison of tertiary treatment by nanofiltration and reverse osmosis for water reuse in denim textile industry’, Journal of Hazardous Materials, Vol.170,no.4, pp. 111-117
Bes-Pia, M, Iborra-Clar, Iborra-Clar, J, Mendoza-Roca, B, Cuartas-Uribe, M 2005, ‘Alcaina-Miranda, Nanofiltration of textile industry wastewater using a physicochemical process as a pre-treatment’, Desalination, vol.178,no.4,pp.343–349.
Biolchi, F, Kawabata & Taylor, J, 2006, ‘Effect of Sulphonation level upon the fixation and build-up properties of reactive dyes.’ Color. Technol, vol.122, no.5, pp.153-156.
Burkinshaw, S, Mignanelli, P, Froehling & Bide 2000, ‘The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton.’ Dyes Pig, vol.47,no.4,pp. 259-267.
Lopes, C,N, J,C,C, Petrus and H 2005, ‘Riella, Color and COD retention by nanofiltration membranes’, Desalination, vol.172,no.2,pp. 77–83.
Ciardelli, G, L, Corsi & M. Marcucci 2000, ‘Membrane separation for wastewater reuse inthe textile industry’, Resources, Conservation Recycling, 31: 189–197.
Ciardelli, G, N, Ranieri 2001, ‘The treatment and reuse of wastewater in the textile industry by means of ozonation and electroflocculation’, Water Res.vol. 35, no.2, pp. 567
Conlon, W & McClellan, S, 1989, ‘Membrane softening: a treatment process comes of age’, Journal of the American Water Works Association, vol.81, no.1, pp.47-51.
Cristiane, N, Lopes, J, Carlos, C, Petrus, H,G 2005, ‘Riella, Colour and COD retention by nanofiltration membranes’, Desalination, vol.172,no. 1,pp. 77–83.
Fu, Z, Zhang, Y & Wang, X 2011, ‘Textiles wastewater treatment using anoxic filter bed and biological wriggle bed-ozone biological aerated filter’, Bioresource Technology, vol. 102, no. 4, pp. 3748-53.
Gould, C 1995, Treating industrial water with membrane technology. Separation and Filtration Systems, vol. 98, no. 6, pp. 38-53.
Gozálvez-Zafrilla,A, Sanz-Escribano, D, Lora-García, J, León Hidalgo, M 2008, Nanofiltration of secondary effluent for wastewater reusein the textile industry Desalination.vol.222,no.3,pp. 272-279.
Hamlin, J, Phillips & Whiting 1999, ‘UV/Visible spectroscopic studies of the effects of common salt and urea upon reactive dye solutions’, Dyes Pigm,vol. 41,no.4,pp.37-142.
Hihara, T, Y, Okada & Z, Morita. 2000, ‘The aggregation of triphenodioxazine reactive dyes in aqueous solution and on cellulosic and nylon substrates’, Dyes Pigm.vol.45, no.5, pp. 131-143.
Hong, S. U., et al. 2006, ‘Removal of dyes, sugars, and amino acids from NaCl solutions using multilayer polyelectrolyte nanofiltration membranes’, Industrial & engineering chemistry research, vol.45,no.18,pp. 6284-6288.
Jakobs, D & Baumgarten, G, 2002, ‘Nanofiltration of nitric acidic solutions from picture tube production’, Desalination, vol. 145, no. 1, pp. 65-8.
Jakobs,D &Baumgarten, G 2002, ‘Nanofiltration of nitric acidic solutions from picture tube production’, Desalination, vol. 145, no. 1, pp. 65-8.
Joshi, M &Purwar, R 2004, ‘Developments in new processes for colour removal from effluent’, Review of Progress in Coloration and Related Topics, vol. 34, no. 1, pp. 58-71.
Judkins Jr, J,F, Hornsby, J 1978, ‘Color removal from textile dye waste using magnesium carbonate,’ J. Water Pollut. Cont. Fed.vol. 50,no.4,pp. 2446.
Kabil, M, S,E, Ghazy 1994, ‘Separation of some dyes from aqueous solutions by flotation’, Sep. Sci. Technol. vol.29,no.8,pp. 25-33.
Kant, R 2012, ‘Textile dyeing industry an environmental hazard’, Natural Science, vol. 4, no. 1, pp. 22-6.
Kim, T-HTH, Park, C, Lee, J, Shin, E-BEB & Kim, S 2002, ‘Pilot scale treatment of textile wastewater by combined process (fluidized biofilm process-chemical coagulation-electrochemical oxidation)’, Water Research, vol. 36, no. 16, pp. 3979-88.
Koyuncu, I, 2002, ‘Reactive dye removal in dye/salt mixtures by nanofiltration membranes containing vinylsulphone dyes; effects of feed concentration and cross flow velocity’, Desalination, vol.143, no.3, pp. 243-253.
Lau, W, & Ismail, A 2009, ‘Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling controls—a review’, Desalination, vol.245.no.1, pp. 321-48.
Libra, JA &Sosath, F 2003, ‘Combination of biological and chemical processes for the treatment of textile wastewater containing reactive dyes’, Journal of Chemical Technology and Biotechnology, vol. 78, no. 11, pp. 1149-56.
Lin, SH & Chen, ML 1997, ‘Treatment of textile wastewater by chemical methods for reuse’, Water Research, vol. 31, no. 4, pp. 868-76.
Marcucci, M, Ciardelli, G, Matteucci, A, Ranieri, L & Russo, M 2002, ‘Experimental campaigns on textile wastewater for reuse by means of different membrane processes’, Desalination, vol. 149, no. 1, pp. 137-43.
Marrot, B, Roche, N 2002, ‘Wastewater treatment and reuse in textile industries,’ a review, Res. Adv. Water Res,vol. 4,no.9,pp. 341–53.
Rautenbach, R & Groeschl, A 1990, ‘Separation Potential of Nanofiltration Membranes’, Desalination,vol. 77,no.1-3,pp. 73-84.
Robinson, T, McMullan, G, Marchant, R & Nigam, P 2001, ‘Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative’, Bioresource Technology, vol. 77, no. 3, pp. 247-55.
Sahinkaya, E, Uzal, N, Yetis, U &Dilek, FB 2008, ‘Biological treatment and nanofiltration of denim textile wastewater for reuse’, Journal of Hazardous Materials, vol. 153, no. 3, pp. 1142-8.
Schaep, J, Van der Bruggen, B, Uytterhoeven, S, Croux, R, Vandecasteele, C, Wilms, D, Van Houtte, E &Vanlerberghe, F 1998, ‘Removal of hardness from groundwater by nanofiltration’, Desalination, vol. 119 no.1,pp. 295-301.
Şen, S &Demirer, GN 2003, ‘Anaerobic treatment of real textile wastewater with a fluidized bed reactor’, Water Research, vol. 37, no. 8, p. 1868.
Tang, C & Chen, V 2002, ‘Nanofiltration of textile wastewater for water reuse’, Desalination, vol. 143, no. 1, pp. 11-20.
Türgay, O, Ersöz, G, Atalay, S, Forss, J &Welander, U 2011, ‘The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation’, Separation and Purification Technology, vol. 79, no. 1, pp. 26-33
Tzitzi, M, Vayenas, D &Lyberatos, G 1994, ‘Pretreatment of textile industry wastewaters with ozone’, Water Science & Technology, vol. 29, no. 9, pp. 151-60.
Van der Bruggen, B &Vandecasteele, C 2003, ‘Removal of pollutants from surface water and groundwater by nanofiltration: overview of possible applications in the drinking water industry’, Environmental Pollution,vol. 122,no.3,pp. 435-45.
van der Graaf,J, & Roorda,J 2000, ‘New developments in upgrading waste-water treatment plant effluent using ultrafiltration’, Membrane Technology, vol.45,no.8,pp. 4- 7.
Verma, AK, Dash, RR &Bhunia, P 2012, ‘A review on chemical coagulation/ flocculation technologies for removal of colour from textile wastewaters’, Journal of environmental management, vol. 93, no. 1, pp. 154-68.
Wang, Z Xue, M Huang, K & Liu, Z 2011, ‘Textile Dyeing Wastewater Treatment’, Huazhong University of Science and Technology China. vol. 68, no. 6, pp. 184-98.
Zaghbani, N, A, Hafiane, M, Dhahbi 2007, ‘Separation of methylene blue from aqueous solution by micellar enhanced ultrafiltration’, Sep. Purif. Technol, vol. 55.no.8, pp. 117–124.
