Introduction
Nuclear power is both liked and loathed because it stands between the border of humanity’s greatest hopes and deepest fears for the future. The fact that atomic energy provides a clean energy alternative that relieves the human race of the problems associated with fossil fuel dependence is not in dispute. However, a mere mention of nuclear energy rekindles the awful memory of the ramifications that were witnessed in the recent Japanese nuclear power plants accident that emitted deadly radioactive steam. Other recent nuclear accidents include the Chernobyl nuclear disaster and the Bhopal nuclear accident. Nuclear energy has elicited emotive debates with some countries considering doing away with its nuclear reactors following the disaster in Japan (Brain and Lamb, 2011). However, very few people are aware of what exactly goes on inside nuclear reactor plants. Very few people, while using electricity in their households, rarely bother to know what goes on between the electric bulbs in their living rooms to the socket, through to the electric power lines all the way to the nuclear power plant.. This essay seeks to highlight a systematic process on how the nuclear reactor works to produce energy, the background, and importance of the process.
History
Statistics state that there are 443 operating nuclear power reactors spread across the planet in 47 different countries and that up to the year 2009, atomic energy accounted for 14% of world’s electricity production. In the United States alone, there 104 nuclear power plants that supply 20% of the electricity that is used in the entire country. Inside a nuclear reactor, a generator helps in producing the spark. The turbine turns the generator. A jet of steam turns the turbine. The radioactive uranium bundle helps in heating water into steam. The water found in the reactor cools the radioactive material hence prevents it from overheating and melting down.
There is no notable difference between coal burning power plants and power plants that depend on atomic energy since both of them heat water into pressurized steam that drives the turbine generator. The only outstanding difference between them is the method used for heating water. Whereas coal-burning power plants burn fossil fuels, nuclear power plants make use of heat that is produced during nuclear fission, a process where an atom splits into two and releases copious amounts of energy. This process occurs naturally in our everyday lives. Uranium, a radioactive element, constantly undergoes spontaneous fission albeit at slow rates hence its ability to emit radiation. This feature makes it the best choice for induced fission that nuclear power plants require. Uranium naturally occurs on earth and has been in existence since beginning of the world. It has several varieties; however, uranium-235(U-235) is the most preferred in nuclear processes. It decays naturally by alpha radiation. In the process, it throws off an alpha particle, that is, two neutrons and two protons that are bound together. Uranium-235 can also undergoes induced fission hence it is suitably used for this process.
Process description of nuclear reactor
When a free neutron is fired into U-235 nucleus, the nucleus absorbs the neutron, become stable, and is split immediately. When the nucleus captures the neutron, it splits into two lighter atoms and throws off two or three new neutrons. This process takes place at a very rapid rate. When a single U-235 atom decays, it releases approximately 200 MeV (million electron volts). This may not seem much too many but it has to be realized that there are a lot more uranium atoms in a pound of uranium. In fact, a pound of highly enriched uranium can be an equivalent of millions of liters of gasoline. When an atom of uranium is split, it releases a high amount of heat and gamma radiations. The two atoms produced after fission later release beta radiation and gamma radiation of their own. For all these to happen, a sample of uranium has to be enriched so that it contains two-to-three percent more U-235. As a matter of fact, 3% enrichment is enough for a nuclear power plant. However, weapon-grade uranium has at least 90 percent U-235 (Brain and Lamb, 2011).
For nuclear fission to be turned into electrical energy, the energy given off by the enriched uranium has to be controlled. This energy is helpful in heating water into steam. Enriched uranium in form of inch long pellets has the same diameter as that of a dime. The pellets are then arranged into long rods that are put together in bundles. The bundles are consequently submerged into water in pressure vessel. The water here acts as a coolant. Without this water, uranium can easily overheat and melt (Brain and Lamb, 2011).
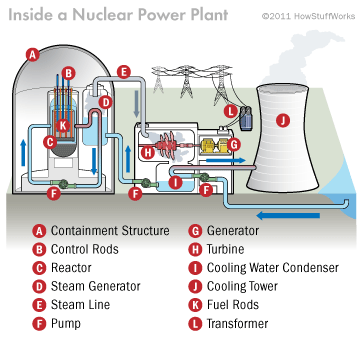
Overheating is normally prevented by putting in place control rods made out of materials capable of absorbing neutrons. These control rods are inserted into uranium bundles by use of a mechanism that can lower or raise them. By raising or lowering the control rods, the operators get to control the rate of the nuclear reaction. When more heat is required, the control rods are lifted out of the uranium bundle. The rods therefore absorb fewer neutrons. The rods are lowered into the uranium bundle to reduce the heat produced. The Uranium bundle produces great amounts of heat energy. It helps in heating water and turns it into steam that drives the turbine. The turbine spins the generator to produce electric power. Some nuclear power plants channels the stem from the reactor through a secondary, intermediate heat exchanger, where a loop of steam water is converted into steam. This drives the turbine. This design is advantageous in that the radioactive water or stem does not contact the turbine. Some reactors also make use of gases like carbon dioxide as the coolant fluid in contact with the reactor core. Liquid metal like sodium or potassium can also be used in place of carbon dioxide as the coolant fluid. The core of such reactors is operated at very high temperatures. Nuclear reactors are divided into three ways of status operation: critical mass, subcritical mass, and supercritical mass. These sections are used by control scientists to determine the stability of the temperature of the core depending on the quality of uranium that is used. Critical and supercritical mass is required for nuclear power plants to raise and lower temperatures with regard to the kilowatts of electricity to be produced. A reactor has a collection of long roods filled with uranium that are bundled together. Other two kinds of nuclear plants also exist. These include fission power plants that make use of breeder reactors and fusion power plants (Brain and Lamb, 2011).
A breeder power plant operates in the same way as light-water power plants except that it uses plutonium instead of uranium as the primary source of fuel. After taking plutonium off the core of the reactor, it can again be reprocessed. After reprocessing and recycling, it is brought back to the nuclear power plant and re-used in the reactor. Every time it is reprocessed, more and more energy is produced. After repeated use, it reaches a point when it can be used no more. Nuclear power plants using plutonium incur lower costs because of the small amount of fuel needed (Oracle, 2011). Reprocessing is also advantageous because it limits the threats that nuclear pose to the environment (Brain and Lamb, 2011).
On the other hand, fusion power plants release absolutely no radiation and this is one of the major advantages of using them. However, very intense heat levels are needed hence it is difficult to use. The energy that is used in running a fusion power plant is the same as that produced by the plants (Oracle, 2011).
Outside the reactor, there is minimal difference with regards to a coal-fired or oil-fired power plant. The only difference is the source of heat used to create steam. This source of heat is capable of emitting harmful levels of radiation hence need for taking extra precaution. A concrete liner houses the reactor’s pressure vessel. This concrete liner is the radiation shield. The concrete liner is housed within a steel containment vessel that contains the reactor core and the equipment that is used in refueling and maintaining the reactor. The steel containment is a barrier that prevents leakage of radioactive gases or fluids from the nuclear power plant. The outer concrete building protects the steel containment vessel. The concrete structure is made in such away that it is capable of surviving damage caused by earthquakes or even crashing jet airliners. The secondary containment structures are pivotal to prevent escape of radiation in case an accident occurs. Their absence in Russian nuclear power plants occasioned the Chernobyl nuclear disaster. Workers in the nuclear power plant control room are at vantage position with respect to taking corrective measures in case anything goes wrong (Brain and Lamb, 2011).
Importance of the processes that take place in the nuclear reactors
A major advantage of nuclear power plants is that they do not depend on fossil fuel and are therefore not affected by fluctuating oil and gas prices. Coal and natural gas power plants have a major disadvantage, that is, the production of carbon dioxide into the atmosphere. Nuclear power plants minimize the amount of carbon dioxide that is emitted into the atmosphere hence minimally contribute to global warming (Falk, 1982). Power that is produced by nuclear plants from the activities that take place within the reactor can produce 2 billion metric tones of carbon dioxide if fossil fuels were instead used. Nuclear power plants that function properly will rarely release radioactive material into the atmosphere relative to coal-fired power plants (Cravens, 2007). Nuclear power stations also have far lower fuel requirements as compared to fossil-fired power stations. Indeed, nuclear fission has a reputation for producing million times more energy per unit weight relative to fossil fuel alternatives (Brain and Lamb, 2011).
Conclusion
Nuclear reactors are a reliable source of energy that are very environmentally friendly because they do not emit carbon dioxide into the atmosphere that contribute to undesirable effects like global warming and other undesirable effects of pollution. However, extra caution has to be taken before nuclear reactors are settled on as sources of energy because of the radioactive elements that it makes use of. Any slight mistake would result to a disaster whose magnitude can be grave than that of Chernobyl and Fukushima.
Reference List
Brain, M. and Lamb, R. (2011). How nuclear power works. Web.
Cravens, G. (2007). Power to Save the World: the Truth about Nuclear Energy. New York: Knopf.
Falk, J. (1982). Global Fission: The Battle over Nuclear Power. Oxford: Oxford University Press.
Oracle. (2011). Nuclear power. Web.
