What is the cost (in the UAE) using PV technology to produce 1 Kg of hydrogen gas?
Fossil fuels are the most common sources of hydrogen, in particular, hydrocarbons such as coal and methane. However, with the increasing requirements of sustainable sources of hydrogen, the electrolysis of water to produce the gas is used. Currently, the process accounts for approximately 4% of the total production of industrial hydrogen. PV technology includes photovoltaic cells encased in an electrolyzer. The cells trap solar energy and convert it into electric energy that powers the breakdown of water by the following equation:
H2O (l) →H2 (g) + ½ O2 (g)
The enthalpy change for the reaction is 285.83 kJ/Mol (Hirsch, 2013).
The above equation applies in 2 grams of hydrogen because the relative atomic mass of 1 mole of hydrogen is 2 grams. To generate 1 kg (1000 g) of hydrogen, the total energy requirements (285.83 kJ/Mol × 1000 g) ÷ 2g = 142,915 kJ/Mol, which is equal to 39.69 kWh. The above reaction assumes that the electrolyzer is working at 100% efficiency. However, the approximate efficiency of a PV module is 65% due to energy losses to the environment.
Actual energy used is (100-65) higher than the calculated, which is 1.35×39.69 =53.5815 kWh.
In the UAE, the current cost of solar energy generated using PV technology is 5.84 cents= $0.0584) per kilowatt-hour (The National, 2016). Therefore, to produce 1 kg of hydrogen gas, the total cost would be 58.5815 × 0.0584, which is equal to $3.42.
How much solar energy is required to produce 1 Kg of hydrogen with current PV and electrolyzer technologies?
The production of hydrogen through PV technology is hampered by the low efficiencies. An electrolyzer with an efficiency of 100% requires 39.69 kWh of electricity to liberate 1 kg of hydrogen (Jacobsson, Fjällström, Edoff, & Edvinsson, 2014). Nevertheless, most efficient electrolyzers attain an efficiency of 65% (Ursua, Gandia, & Sanchis, 2012). This occurrence implies that an additional 35% of the original energy requirements is needed. The total energy is 1.35 × 39.69 kWh= 58.5815 kWh.
How much energy can be derived from 1 Kg of hydrogen if converted back to electricity via a fuel cell?
The conversion of hydrogen into electricity in a fuel cell involves a chemical reaction that is opposite to the one that occurs during electrolysis. The reaction liberates 237.13 kJ/Mol in an ideal setting, which translates to 32.934 kWh for 1 kg of hydrogen and an efficiency of 83%. However, according to Garche et al. (2013), modern fuel cells operate at 60% efficiency.
If 32.934 kWh represents 83% efficiency, the energy production at 60% efficiency = (60×32.934) ÷ 83= 23.8077 kWh.
With current efficiencies and costs of solar panels, is it feasible in this region?
The current cost of solar panels in this region has been approximated at 4 cents per kWh (Fehrenbacher, 2016). However, the efficiencies of the fuel cells negate the benefits of cheap solar energy by generating low energy when hydrogen from PV cells is converted back to electricity. To find the value of the generated power in comparison with conventional electricity prices, it is important to compare the cost of generated energy to current electricity prices. Currently, 1 kWh of electricity costs 0.23 AED (0.06 U.S.) (Dubai Electricity and Water Authority, 2016).
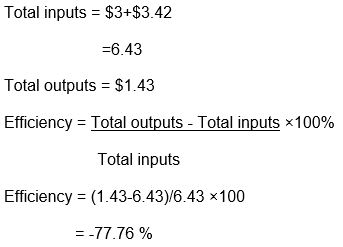
The 23.8077 kWh from burning 1 kg of hydrogen= 23.8077× 0.06 = $1.43 per kg of hydrogen. With the current cost of production of $3.42, even without considering the initial cost of the systems, the process is not feasible because the outputs are less than the input.
The efficiency of all components of the system
Total inputs = Cost of PV cell per kWh+ cost of producing 1 kg of hydrogen
The cheapest solar module costs $3 per peak watt.
The generation of hydrogen using PV technology and the subsequent regeneration of electricity from the combustion of hydrogen in a fuel cell is not efficient in the UAE. Even though the cost of solar energy has substantially dropped throughout the years, it appears that the low efficiencies of the equipment reduce the final electricity output when hydrogen is burned in a fuel cell.
References
Dubai Electricity & Water Authority. (2016). Tariff calculator. Web.
Fehrenbacher, K. (2016). A jaw-dropping world record solar price was just bid in Abu Dhabi. Web.
Garche, J., Dyer, C. K., Moseley, P. T., Ogumi, Z., Rand, D. A. J. & Scrosati, B. (2013). Bruno encyclopedia of electrochemical power sources. The Netherlands: Elsevier.
Hirsch, R. (2013). Hydrogen and fuel cells. North Mankato, Minnesota: ABDO Publishing Company.
Jacobsson, T. J., Fjällström, V., Edoff, M., & Edvinsson, T. (2014). Sustainable solar hydrogen production: From photoelectrochemical cells to PV-electrolyzers and back again. Energy & Environmental Science, 7(7), 2056-2070.
Mahapatra, S. (2016). Dubai gets record-low bid of 2.99¢/kWh for 800 MW solar PV project. Web.
The National. (2016). UAE beats renewables cost hurdle with world’s cheapest price for solar energy. Web.
Ursua, A., Gandia, L. M., & Sanchis, P. (2012). Hydrogen production from water electrolysis: Current status and future trends. Proceedings of the IEEE, 100(2), 410-426.
